Implementation of Saliva-Check Mutans bacterial identification kit as educational aid for patients during pre-natal dental visits
Primary Investigator: Dr. Joshua Thomson (thomsojo@udmercy.edu)
Co-Investigator: Erin Relich, Professor of Dental Hygiene (relichee@udmercy.edu)
Project Support: Dr. John Girdwood (girdwojr@udmercy.edu)
Training Material
- Research Slide Deck (PPT)
- Research Slide Deck (PDF)
- Maternal Oral Health Handout
- Maternal Oral Health Hygienist Checklist (Protocol)
- VIDEO: Saliva-Check Mutans Kit “How To”
Study Material
Important Note…
Do NOT click the links below unless you are a trained hygienist participating in this study!
Pre-Natal
- Note: Clicking the survey link, whether below or however you access the survey, will create a blank record in the data set so please only click the link when absolutely necessary and/or when seeing a patient to do a real live survey response; thanks!
- Before clicking the active survey link below, take a moment to view a PDF of the survey (Click here for Prenatal Survey PDF)
- Initial Screen Survey

Post-Natal
- Note: Clicking the survey link, whether below or however you access the survey, will create a blank record in the data set so please only click the link when absolutely necessary and/or when seeing a patient to do a real live survey response; thanks!
- Before clicking the active survey link below, take a moment to view a PDF of the survey (Click here for postnatal survey PDF)
- Post-Delivery Survey

Thank you to all our hygienists and patients!
FAQ
Here are some questions that our hygienists have asked and the answers might also help you!
Q: What gestation week would be too late to include the patient in the research?
A: If patient consents, any week is fine! Check out this screen shot of the survey that shows how we break up the weeks and include all terms of pregnancy in this study.
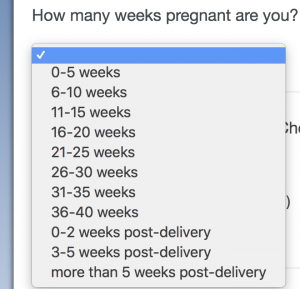
Q: What is the “research number” and how do I create one for a patient?
A: Each site was assigned a “prefix” letter which we also call a “site code” and Dr. Girdwood has those so contact him (girdwojr@udmercy.edu) if you need a reminder but do not contact Dr. Thomson to get your code because he (Dr. Thomson) cannot see those numbers; they are kept away from Dr. T to maintain study anonymity for participants. Let’s say your site code is “Z.” You would simply see the first patient and give her a number like “Z1” or “Z100” – something easy for you to remember – and, then keep going sequentially for subsequent patients like Z101, Z102, Z103, etc. It is very important that you – the hygienist at the site – know that number! Each patient will be seen again, as you know, after the child is born and you will use the same patient number for that post-natal visit so you NEED to remember the patient number for the patient!
Q: What if the patient declines the saliva test? Do I assign her a patient number?
A: This is a great question but let’s first back up a step. If the patient declines to sign the consent form, i.e. she declines to participate in the study, then STOP!!! Again, stop there! In the unlikely situation where the patient consents to participate, signs the consent form, but then declines to have the saliva test… well, you can proceed through the study protocol and assign her a patient number. But, that’s probably unlikely. Remember, if the patient declines to participate: STOP!
Q: We didn’t receive the saliva tests… Where are the saliva tests?
A: These were mailed in December and sites have received them. If your site did not receive the saliva tests, contact Dr. Josh Thomson immediately at thomsojo@udmercy.edu
Q: What is up with all the redundant questions? Paper and online surveys?
A: This project started swiftly and we created some instruments (research survey and paper assessment tool) very quickly! Our first drafts included some redundancy and, although we tried very hard to avoid that, it is present in our first and second drafts. If you see something specific that is excessive, confusing, redundant, or otherwise goofy – please send an email to Dr. T. (thomsojo@udmercy.edu) and Dr. G. (girdwojr@udmercy.edu) so that we can both adjust the study and standard work accordingly. Some things cannot be changed because they are “firmly set” by the research protocol but many things can be changed and we highly encourage your feedback because the success of this project rests on your input! Essentially, the paper instrument and the research online survey were created separately. If you see something on both, we can probably remove it from the paper tool (and, keep it on the research online survey). Some other things on the paper tool are going to be recorded on your EHR/EDR so we can probably trim those off the paper eventually, too! Again, let us know your thoughts and we can improve!
Q: How can we integrate the survey into our EDR/EHR?
A: One of our brilliant administrators at a pilot site has a nifty way of placing web links directly to the research survey and other relevant info directly on the EDR/EHR screen.

This bar is at the top of the computer screen when a hygienist is providing care to a patient. The provider (hygienist) clicks the “Link” icon at the top of the computer screen and, then…
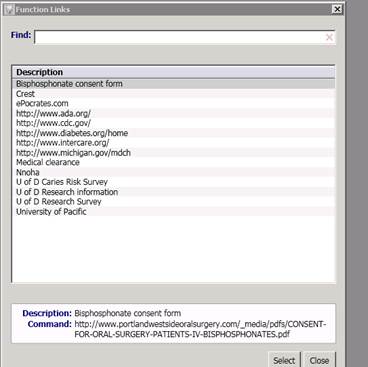
The links appear in a drop-down box for easy access!
Q: How often should we mail the consent forms to Josh?
A: It is best to give signed consent forms to your Regional Coordinator during every site visit.
Q: Should we scan the consent form into the EHR/EDR?
A: Scanning is not required but it would be helpful. We know that your are busy and scanning adds work so please think about what your site is capable of and go from there!
Q: Let’s say a patient has already had a cleaning but it was prior to the beginning of the research project. Should we still do a saliva test if the hygienist happens to catch the patient at a later date in their pregnancy no matter what week they’re at in their pregnancy?
Yes! There is a space in the research survey that asks what week gestation and we will parse those out in the data when that time comes. But, the more the merrier!
Q: Is the gum (chewing wax) necessary for the study if they have enough saliva?
Yes, the gum is necessary. It helps to remove plaque bacteria to get a better estimate of the total S. mutans
Q: A lot of lines are faint. Is there any correlation if the line comes up quickly, does it mean more S. mutans?
There is some correlation; faint band is possibly a bit lower. However, the manufacturer states that any band regardless of intensity would be considered “caries risk.” If it is faint, just check the “weakly positive” box.
Q: Do you prefer the patient to the survey alone? Or, go over it with the patient?
The hygienist should input the research number and the result of the test. If the patient feels comfortable doing it on their own, that would be great. If they need assistance with interpretation or reading then please help out however you can. We’ll leave that to your discretion.
Q: How long can we wait for the post-op?
The post-partum survey and S. mutans test should be administered after the women deliver and come back to the clinic for their checkup. This is the important one! Earmark the patients that did the initial survey to make sure that you know when they are coming back to get the second test and survey completed.
Q: If the patient just delivered, and is pregnant again, is it 1st trimester or post-op? Or both?
The post-partum test and survey are only to be done if a patient has completed the first test and survey (and signed the Informed Consent). If they took it during the first pregnancy and are now back they can take the post-partum test and survey.
Q: Referral to a different DDS… still mark yes in the codes?
Yes! Of course, this refers to the paper intake form (click here).
Q: If we see a child of an OB patient on a different day, where should we put the info for tracking the child was seen?
If you’re using the paper form, you can still mark it on the same row as the patient’s last visit. The date really isn’t important. We are more interested in counting the number of children who we serve through the project. Therefore, you can put it on the (mother’s) paper form on the row of the most recent visit. Of course, you need to drop the code for the child!
Sensitivity to Patient Circumstances
Minors
Omit minors from the research study. In the project description it is stated “volunteers in the study will be adult females of childbearing age.” Therefore, the “adult female” criteria excludes any participation of minors.
Patients with Dentures
Of course, you want to provide the patient with all requested services (especially education and ensuring patient has a dental home) but we are still thinking about how to best serve these patients and, separately, how to incorporate them as research participants if at all appropriate.
Adoption
If a mother you are caring for is partnering with a new adoptive family, it may be difficult to approach the subject of transferring bacteria to the child. In this case, if mom consents to participate in the study, the best scenario (based on suggestions from a hygienist that faced this scenario) would be to stress that dental care is important for the patient regardless. If you are guiding a mother through the survey in this circumstance , please feel free to omit (by leaving blank) the maternal-child transfer of bacteria questions because we do not want to make the mother uncomfortable or uneasy in any way. We will still get the information we need without those questions answered. Also, feel free to use discretion with your explanation about bacteria and dental disease in terms of maternal-child transfer. Additionally, remember that paraphrasing the survey questions is up to your discretion.
Life Changes
Every patient has a different pregnancy experience and many of our patients will undergo significant life changes during this very momentous period of life. We may encounter the following situations: patient is no longer a patient at the site, changed provider, or had a miscarriage. Clinicians may wonder what the proper protocol is for these patients and clinicians might have questions like “Are these patients still counted in the data pull? Should the RDH follow up?” Of course, our primary concern is the patient’s well-being! Each OB clinical site should have a clinical protocol already in place regarding how to care for these patients in these situations. The RDH should connect with the OB clinical team to come to learn and understand best practices for providing patient-centered care in any situation.
There are several ways a patient may end a relationship with a clinical team. She might just decide to go elsewhere and that is her choice. She may change provider. And, some times, the mother might have a miscarriage which would result in her no longer visiting the OB clinic (after a final visit). For all of these instances, we will lose our connection with the patient. We cannot maintain ties with every patient. We can only hope that our first interaction involved a referral that prompted the patient to establish a dental home. If that happened, then we will continue to see the patient! If the patient did not establish a dental home and ends the relationship with the OB clinic, e.g. she changes providers or has a miscarriage, we will also end our relationship with the patient for purposes of this project.